Download fasta file
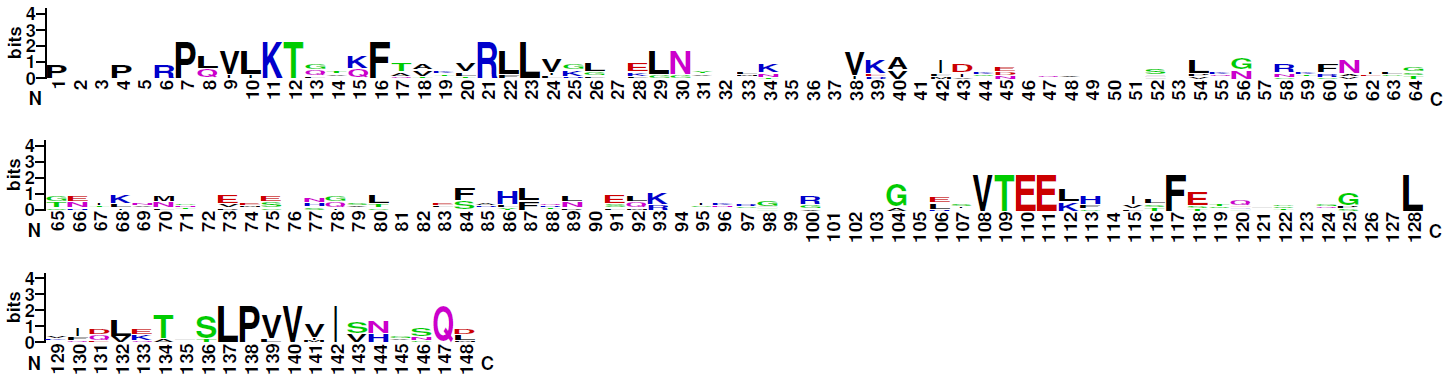
Blue: basic residues (KRH); red: acidic residues (DE); purple: amides (QN); black: hydrophobic residues (AVLIPWFM); green: polar (CSTYG) (http://weblogo.berkeley.edu/)
| 6.2 | Class: STAT domain factors (STAT) | |
| Description: | Class description: STAT proteins bind to DNA as dimers. The DNA-contacting interface is organized by an eight-stranded beta-barrel, N-terminally preceded by a four-helix bundle and C-terminally followed by a mostly alpha-helical connector region. The residues that bind to the major groove of the DNA are mostly exposed by loops connecting the beta-strands of the beta-barrel and the one linking the beta-barrel and the first helix of the 'connector' region. The STAT dimer nearly completely embraces the DNA double helix, compared with a 'pair of pliers' (PMID 9671298), with the DNA-binding interface as jaws and the four-helix bundles as handles. Bound by STAT, the DNA undergoes a moderate bending of about 40 degrees. (PMID 9671298) | |
| Aligned domain sequences: | Alignment of STAT domains Download fasta file |
|
| Logo plot: |  Blue: basic residues (KRH); red: acidic residues (DE); purple: amides (QN); black: hydrophobic residues (AVLIPWFM); green: polar (CSTYG) (http://weblogo.berkeley.edu/) |
|